P0710S, Rapid PNGase F - 50 reactions
Rapid PNGase F enables complete and rapid deglycosylation of antibodies and immunoglobulin fusion proteins, as well as other glycoproteins, in minutes. All N-glycans are released rapidly and without bias, and are ready to be prepared for downstream chromatography or mass spectrometry analysis. Rapid PNGase F creates an optimized workflow, reducing processing time without compromising sensitivity or reproducibility.
Deglycosylation in minutes for N-glycan analysis
Rapid PNGase F enables complete and rapid deglycosylation of antibodies and immunoglobulin fusion proteins, as well as other glycoproteins, in minutes. All N-glycans are released rapidly and without bias, and are ready to be prepared for downstream chromatography or mass spectrometry analysis. Rapid PNGase F creates an optimized workflow, reducing processing time without compromising sensitivity or reproducibility.
- Complete deglycosylation of antibodies and immunoglobulin fusion proteins in 10 minutes
- Recombinant enzyme; guaranteed endoglycosidase F1, F2 or F3 free
- All reaction components are compatible with HPLC and mass spectrometry analysis
- ≥ 95% purity, as determined by SDS-PAGE and intact ESI-MS
- Optimal activity and stability ensured for up to 12 months
- Fast reaction setup
Application Features
Rapid PNGase F is an improved recombinant reagent that allows the complete and rapid deglycosylation of antibodies and fusion proteins in only minutes. All N-glycans are released rapidly and without bias, ready to be prepared for downstream chromatography or mass spectrometry analysis.
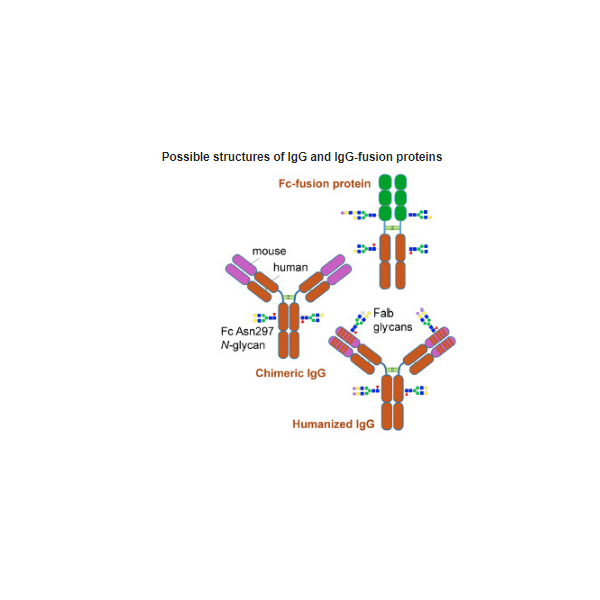
A variety of therapeutic monoclonal antibodies were used to validate Rapid PNGase F: different subclasses (IgG 1 to 4), isotypes (IgA, IgM, IgE), organisms (mouse, human, and humanized), sources (CHO, murine myeloma), and structures (IgG, IgG-fusions).
Rapid PNGase F can effectively remove all N-glycans from both conserved (i.e. Fc Asn297) and non-conserved (i.e. Fab N-glycans) glycosylation sites. Validation was in accordance with published data. Sensitivity and specificity are not compromised by a faster and more convenient glycoprotein characterization workflow using Rapid PNGase F.
| Price | 3.222,00 RON (preturile sunt fara TVA) |
|---|---|
| Description |
Deglycosylation in minutes for N-glycan analysis Rapid PNGase F enables complete and rapid deglycosylation of antibodies and immunoglobulin fusion proteins, as well as other glycoproteins, in minutes. All N-glycans are released rapidly and without bias, and are ready to be prepared for downstream chromatography or mass spectrometry analysis. Rapid PNGase F creates an optimized workflow, reducing processing time without compromising sensitivity or reproducibility.
Application Features Rapid PNGase F is an improved recombinant reagent that allows the complete and rapid deglycosylation of antibodies and fusion proteins in only minutes. All N-glycans are released rapidly and without bias, ready to be prepared for downstream chromatography or mass spectrometry analysis. |

 English
English