P0722L, alpha 2-3,6,8,9 Neuraminidase A - 4.000 units
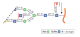
α2-3,6,8,9 Neuraminidase A is a broad specificity sialidase, which cleaves linear and branched non-reducing terminal sialic acid residues from glycoproteins, glycopeptides, and oligosaccharides. It can be used for glycan analysis and characterization and intact glycoprotein remodeling, in vitro and in vivo.
α2-3,6,8,9 Neuraminidase A is a broad specificity sialidase, which cleaves linear and branched non-reducing terminal sialic acid residues from glycoproteins, glycopeptides, and oligosaccharides. It can be used for glycan analysis and characterization and intact glycoprotein remodeling, in vitro and in vivo.
- Recombinant enzyme with no detectable endoglycosidase or other exoglycosidases contaminating activities
- Acts on both Neu5Ac and Neu5Gc
- Double digest with other exoglycosidases and endoglycosidases
- Tolerant of moderate levels (0.5-1.0%) of detergents
- ≥95% purity, as determined by SDS-PAGE and intact ESI-MS
- Optimal activity and stability for up to 24 months
Product Source
Cloned from Arthrobacter ureafaciens and expressed in E. coli.
| Price | 2.004,00 RON (preturile sunt fara TVA) |
|---|---|
| Description |
α2-3,6,8,9 Neuraminidase A is a broad specificity sialidase, which cleaves linear and branched non-reducing terminal sialic acid residues from glycoproteins, glycopeptides, and oligosaccharides. It can be used for glycan analysis and characterization and intact glycoprotein remodeling, in vitro and in vivo.
Product Source Cloned from Arthrobacter ureafaciens and expressed in E. coli. |

 English
English