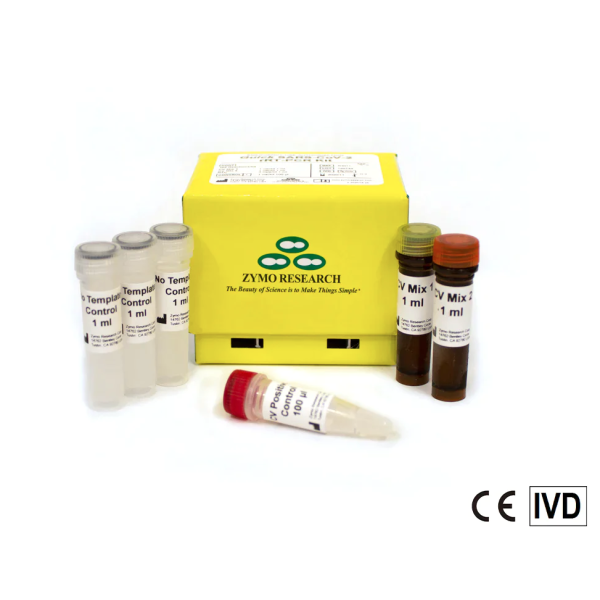
R3011-10K, Quick SARS-CoV-2 rRT-PCR Kit (10,000 rxns)
The Quick SARS-CoV-2 rRT-PCR Kit is a real-time reverse transcription PCR (rRT-PCR) test for the qualitative detection of RNA from the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) which is responsible for the coronavirus disease (COVID-19).
The Quick SARS-CoV-2 rRT-PCR Kit detects N1, N2, and N3 regions of the viral nucleocapsid (N) gene and a host-specific target region (human RNase P gene) to assess sample quality. The kit also includes CV Positive Control that enable assay performance monitoring, and a No-Template Control to confirm the absence of contamination in the reagents.
The Quick SARS-CoV-2 rRT-PCR Kit can be used on purified RNA samples isolated from upper respiratory and lower respiratory systems.
The Quick SARS-CoV-2 rRT-PCR Kit has High Sensitivity, with a Limit of Detection as low as 15 viral genome equivalent copies per reaction, a fast turnaround time of less than 1.5 hours, and a simple workflow in which RNA is simply added to the Quick SARS-CoV-2 rRT-PCR Kit reagents and directly analyzed.
Due to its simplicity, set-up up can be performed in an automation and it is compatible with high-throughput platforms. The kit is compatible with multiple Real-Time PCR instruments and technical support is available for all steps of the setup process and data interpretation.
HIGHLIGHTS
- Approved for emergency use authorization (EUA) by the U.S. Food and Drug Administration and CE-IVD certified.
- High Sensitivity: Ranked among the top three most sensitive EUA tests by the FDA, with a Limit of Detection as low as 250 GEC/ml (15 GEC/reaction).
- Specific: Detection of SARS-CoV-2 and Emerging Strains (including Omicron)
- Rapid & Easy Setup: Ready-to-use Master Mix, just add sample.
- Compatible with Automated and High-Throughput workflows.
DESCRIPTION
TECHNICAL SPECIFICATIONS
| Compatibility | Compatible with automated and high-throughput workflows. |
|---|---|
| Equipment Required | Real-Time PCR Instruments capable of detecting HEX™ and Quasar® 670/Cy5 fluorophores. |
| Input Quality | Purified RNA free of enzymatic inhibitors. |
| Processing Time | ≤ 1.5 hours from set up to results. |
| Reagents | Complete and ready to use master mixes. |
| Registration Status | FDA-Emergency Use Authorization CE-IVD certified |
| Sample Input Material | Purified RNA samples isolated from upper respiratory and lower respiratory systems. |
| Supplemental Info |
Mai multe informatii
| Price | 929.735,00 RON (preturile sunt fara TVA) | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Description |
HIGHLIGHTS
DESCRIPTIONTECHNICAL SPECIFICATIONS
|

 English
English