E8212S, Ph.D.-C7C Phage Display Peptide Library Kit v2 - 10 Panning Experiments
The Ph.D.-C7C Phage Display Peptide Library Kit v2 is based on a combinatorial library of random disulfide looped peptides fused to the N-terminus of a minor coat protein (pIII) of M13 phage. The library is displayed in the form AC-X7-C-GGG, which directly precedes the wild-type pIII sequence.
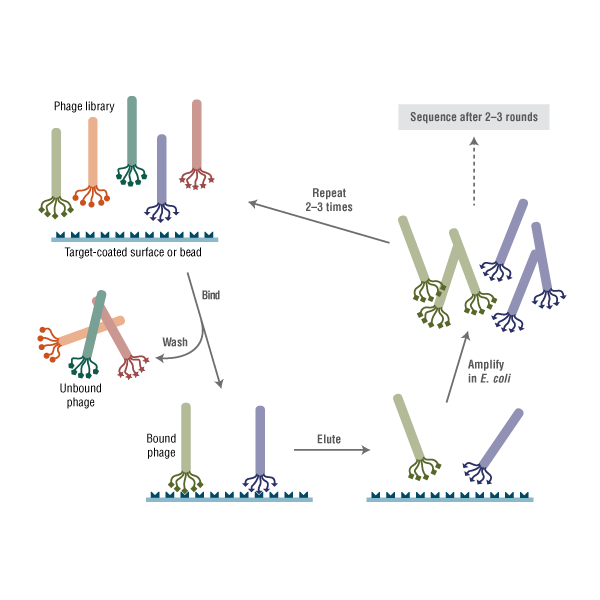
Phage display describes a selection technique in which a library of peptide or protein variants is expressed on the outside of a phage virion, while the genetic material encoding each variant resides on the inside. This creates a physical linkage between each variant protein sequence and the DNA encoding it, which allows rapid partitioning based on binding affinity to a given target molecule (antibodies, enzymes, cell-surface receptors, etc.) by an in vitro selection process called panning. In its simplest form, panning is carried out by incubating a library of phage-displayed peptides on a plate (or bead) coated with the target, washing away the unbound phage, and eluting the specifically bound phage. The eluted phage are then amplified and taken through additional binding/amplification cycles to enrich the pool in favor of binding sequences. After 3–4 rounds, individual clones are characterized by DNA sequencing and binding assays.
The Ph.D.-C7C Phage Display Peptide Library Kit v2 is based on a combinatorial library of random disulfide looped peptides fused to the N-terminus of a minor coat protein (pIII) of M13 phage. The library is displayed in the form AC-X7-C-GGG, which directly precedes the wild-type pIII sequence. Under neutral conditions disulfide loop formation is favored. Interest in disulfide looped libraries is based on the chance that imposing structural constraints on an unbound ligand may result in a less unfavorable binding entropy upon target binding relative to an unconstrained library. The library consists of ~109 electroporated sequences amplified once to yield approximately 100 copies of each sequence in 10 μl of the supplied phage.
Mai multe informatii
| Price | 5.400,00 RON (preturile sunt fara TVA) |
|---|---|
| Description |
Phage display describes a selection technique in which a library of peptide or protein variants is expressed on the outside of a phage virion, while the genetic material encoding each variant resides on the inside. This creates a physical linkage between each variant protein sequence and the DNA encoding it, which allows rapid partitioning based on binding affinity to a given target molecule (antibodies, enzymes, cell-surface receptors, etc.) by an in vitro selection process called panning. In its simplest form, panning is carried out by incubating a library of phage-displayed peptides on a plate (or bead) coated with the target, washing away the unbound phage, and eluting the specifically bound phage. The eluted phage are then amplified and taken through additional binding/amplification cycles to enrich the pool in favor of binding sequences. After 3–4 rounds, individual clones are characterized by DNA sequencing and binding assays. |

 English
English