P0707L, PNGase A - 750 units
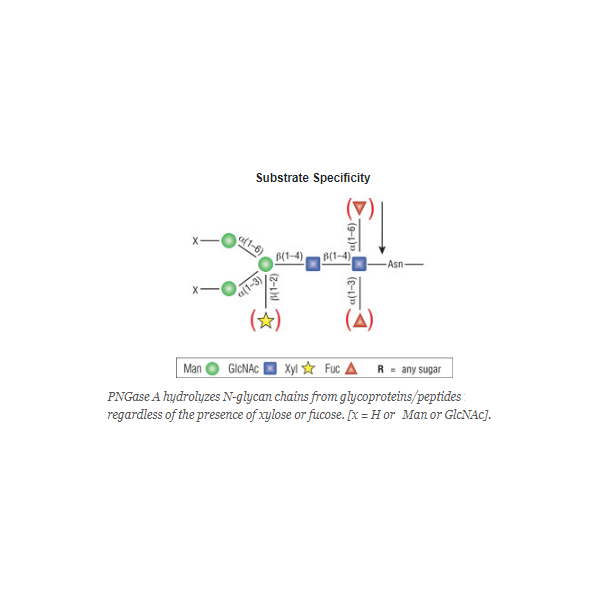
PNGase A cleaves between the innermost GlcNAc and asparagine residues of high mannose, hybrid, and short complex oligosaccharides such as those found in plant and insect cells from N-linked glycoproteins and glycopeptides. PNGase A differs from PNGase F in that it cleaves N-linked glycans with or without α(1,3)-linked core fucose residues.
PNGase A cleaves between the innermost GlcNAc and asparagine residues of high mannose, hybrid, and short complex oligosaccharides such as those found in plant and insect cells from N-linked glycoproteins and glycopeptides. PNGase A differs from PNGase F in that it cleaves N-linked glycans with or without α(1,3)-linked core fucose residues.
- Cleaves N-glycans from plant and insect derived glycoproteins and glycopeptides
- Activity is not inhibited by an α1-3 Fucose residue on the chitobiose core
- Recombinant enzyme with no detectable exoglycosidase or other endoglycosidase contaminating activities
- Glycerol-free for optimal performance in HPLC and mass spectrometry analysis
- ≥95% purity, as determined by SDS-PAGE and intact ESI-MS
- Optimal activity and stability for up to 24 months
Product Source
Cloned from Oryza sativa (rice) and expressed in Pichia pastoris.
| Price | 7.614,00 RON (preturile sunt fara TVA) |
|---|---|
| Description |
PNGase A cleaves between the innermost GlcNAc and asparagine residues of high mannose, hybrid, and short complex oligosaccharides such as those found in plant and insect cells from N-linked glycoproteins and glycopeptides. PNGase A differs from PNGase F in that it cleaves N-linked glycans with or without α(1,3)-linked core fucose residues.
Product Source Cloned from Oryza sativa (rice) and expressed in Pichia pastoris. |

 English
English