M0299S, Endonuclease VIII - 1.000 units
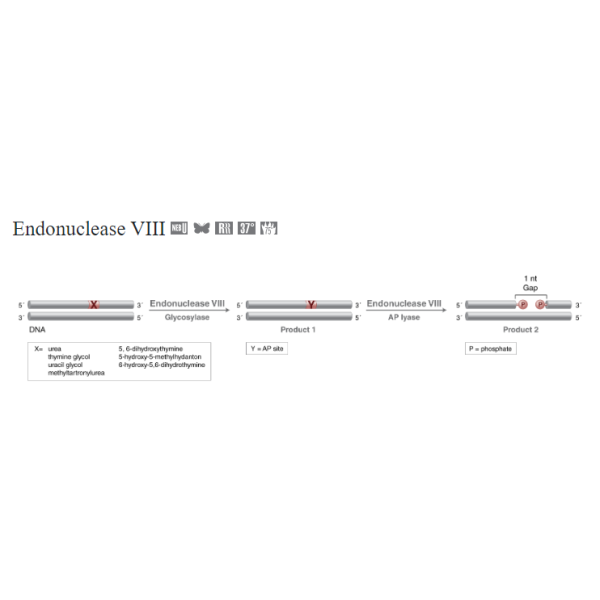
Endonuclease VIII from E. coli acts as both an N-glycosylase and an AP-lyase. The N-glycosylase activity releases damaged pyrimidines from double-stranded DNA, generating an apurinic (AP site). The AP-lyase activity cleaves 3´ and 5´ to the AP site leaving a 5´ phosphate and a 3´ phosphate
Endonuclease VIII from E. coli acts as both an N-glycosylase and an AP-lyase. The N-glycosylase activity releases damaged pyrimidines from double-stranded DNA, generating an apurinic (AP site). The AP-lyase activity cleaves 3´ and 5´ to the AP site leaving a 5´ phosphate and a 3´ phosphate. Damaged bases recognized and removed by Endonuclease VIII include urea, 5, 6- dihydroxythymine, thymine glycol, 5-hydroxy-5- methylhydantoin, uracil glycol, 6-hydroxy-5, 6-dihydrothymine and methyltartronylurea. While Endonuclease VIII is similar to Endonuclease III, Endonuclease VIII has β and δ lyase activity while Endonuclease III has only β lyase activity.
Product Source
An E. coli strain which carries the cloned nei gene.
| Price | 534,00 RON (preturile sunt fara TVA) |
|---|---|
| Description |
Endonuclease VIII from E. coli acts as both an N-glycosylase and an AP-lyase. The N-glycosylase activity releases damaged pyrimidines from double-stranded DNA, generating an apurinic (AP site). The AP-lyase activity cleaves 3´ and 5´ to the AP site leaving a 5´ phosphate and a 3´ phosphate. Damaged bases recognized and removed by Endonuclease VIII include urea, 5, 6- dihydroxythymine, thymine glycol, 5-hydroxy-5- methylhydantoin, uracil glycol, 6-hydroxy-5, 6-dihydrothymine and methyltartronylurea. While Endonuclease VIII is similar to Endonuclease III, Endonuclease VIII has β and δ lyase activity while Endonuclease III has only β lyase activity. Product SourceAn E. coli strain which carries the cloned nei gene. |

 English
English