M0247S, Micrococcal Nuclease - 320.000 gel units
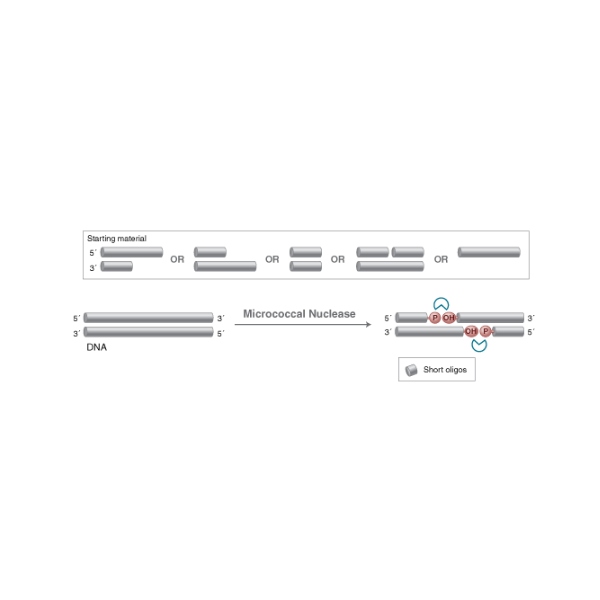
Micrococcal nuclease is derived from Staphylococcus aureus and is a relatively non-specific endo-exonuclease. It is purified from a recombinant E. coli strain that digests double-stranded, single-stranded, circular and linear nucleic acids.
Micrococcal nuclease is derived from Staphylococcus aureus and is a relatively non-specific endo-exonuclease. It is purified from a recombinant E. coli strain that digests double-stranded, single-stranded, circular and linear nucleic acids. The enzyme is active in the pH range of 7.0 - 10.0, with optimal activity at pH 9.2 for both RNA and DNA substrates. Cleavage preferences have been observed at sites rich in adenylate, deoxyadenylate or thymidylate. Both DNA and RNA are degraded to 3´ phosphomononucleotides and dinucleotides.
Micrococcal Nuclease works well with the SimpleChIP® Enzymatic Chromatin IP Kit (Magnetic Beads) #9003 from Cell Signaling Technology. Please follow the link to see product information and protocols on their website.
Product Source
An E. coli strain containing a genetic fusion of the micrococcal nuclease gene (Gene ID: 3238436) and the gene coding for maltose binding protein, or MBP. The micrococcal nuclease is cleaved from the fusion protein and purified away from MBP.
| Price | 528,00 RON (preturile sunt fara TVA) |
|---|---|
| Description |
Micrococcal nuclease is derived from Staphylococcus aureus and is a relatively non-specific endo-exonuclease. It is purified from a recombinant E. coli strain that digests double-stranded, single-stranded, circular and linear nucleic acids. The enzyme is active in the pH range of 7.0 - 10.0, with optimal activity at pH 9.2 for both RNA and DNA substrates. Cleavage preferences have been observed at sites rich in adenylate, deoxyadenylate or thymidylate. Both DNA and RNA are degraded to 3´ phosphomononucleotides and dinucleotides. Product Source An E. coli strain containing a genetic fusion of the micrococcal nuclease gene (Gene ID: 3238436) and the gene coding for maltose binding protein, or MBP. The micrococcal nuclease is cleaved from the fusion protein and purified away from MBP. |

 English
English