M0206L, Exonuclease III - 25.000 units
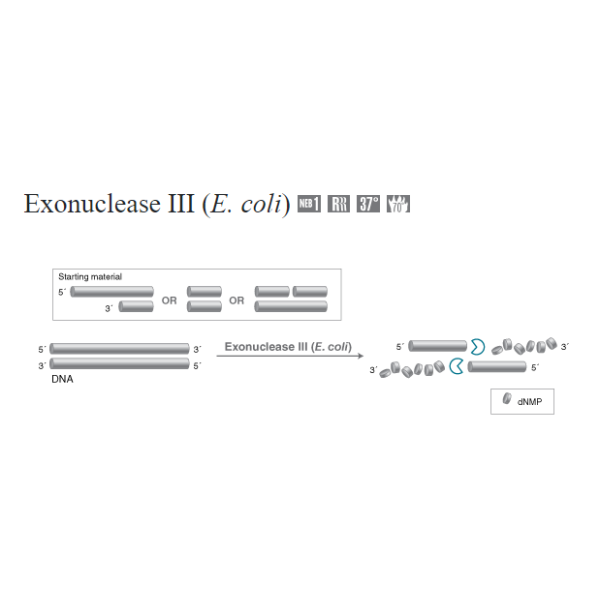
Catalyzes the stepwise removal of mononucleotides from 3´-hydroxyl termini of duplex DNA. A limited number of nucleotides are removed during each binding event, resulting in coordinated progressive deletions within the population of DNA molecules. Exonuclease III has also been reported to have RNase H, 3´-phosphatase and AP-endonuclease activities.
Product Information
Catalyzes the stepwise removal of mononucleotides from 3´-hydroxyl termini of duplex DNA. A limited number of nucleotides are removed during each binding event, resulting in coordinated progressive deletions within the population of DNA molecules. Exonuclease III has also been reported to have RNase H, 3´-phosphatase and AP-endonuclease activities.
The preferred substrates are blunt or recessed 3´-termini, although the enzyme also acts at nicks in duplex DNA to produce single-strand gaps. 3´-protruding termini are resistant to cleavage; the degree of resistance depends on the length of the extension,with extensions 4 bases or longer being essentially resistant to cleavage.
Exonuclease III activity depends partially on helical structure and displays sequence dependence (C>A=T>G). Temperature, salt concentration and the ratio of enzyme to DNA greatly affect enzyme activity, requiring reaction conditions to be tailored to specific applications.
Double-stranded DNA specific exonuclease
Initiates at the 3' termini of linear double-stranded DNA with 5' overhangs or blunt ends and 3' overhangs containing less than four bases
Initiates at nicked sites in double-stranded DNA
Catalyzes the removal of nucleotides from linear or nicked double-stranded DNA in the 3' to 5' direction
Exonuclease III is ideal for:
Site-directed mutagenesis
Preparation of single-stranded DNA for dideoxy sequencing
Preparation of nested deletions in double-stranded DNA
Product Source
An E. coli strain that carries the cloned Exonuclease III gene from E. coli.
| Price | 1.776,00 RON (preturile sunt fara TVA) |
|---|---|
| Description |
Product Information The preferred substrates are blunt or recessed 3´-termini, although the enzyme also acts at nicks in duplex DNA to produce single-strand gaps. 3´-protruding termini are resistant to cleavage; the degree of resistance depends on the length of the extension,with extensions 4 bases or longer being essentially resistant to cleavage. Exonuclease III activity depends partially on helical structure and displays sequence dependence (C>A=T>G). Temperature, salt concentration and the ratio of enzyme to DNA greatly affect enzyme activity, requiring reaction conditions to be tailored to specific applications.
Exonuclease III is ideal for: Site-directed mutagenesis
|

 English
English