E5510S, Gibson Assembly Cloning Kit - 10 rxns
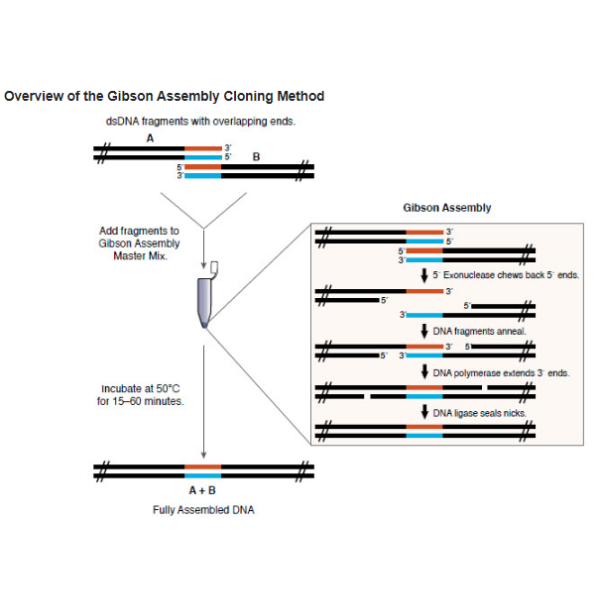
Gibson Assembly® allows for successful assembly of multiple DNA fragments, regardless of fragment length or end compatibility.
Gibson Assembly® allows for successful assembly of multiple DNA fragments, regardless of fragment length or end compatibility.
- Assembly and transformation in just under two hours
- Flexible sequence design (scar-less cloning)
- No PCR clean-up step required
- High transformation efficiencies for inserts up to 20 kb
- Easily adapted for multiple DNA manipulations, including site-directed mutagenesis
- Includes competent cells
Specification:
10 μl of 2X Gibson Assembly Master Mix was incubated with 6 fragments (5 fragments of 400 bp and one of 2,780 bp, with 40 bp overlap, 0.05 pmol each) in a final volume of 20 μl at 50°C for 60 minutes. NEB 5-alpha Competent E. coli (NEB #C2987) were transformed with 2 μl of the master mix/fragment mixture using the transformation protocol on page 12. Greater than 100 white colonies were observed when 1/10 of the outgrowth was spread on an ampicillin plate with IPTG/Xgal and incubated overnight.
Overview of Gibson Assembly Cloning Kit Protocol:
- Design primers to amplify fragments (and/or vector) with appropriate overlaps
- PCR amplify fragments using a high-fidelity DNA polymerase.
- Prepare linearized vector by PCR amplification using a high-fidelity DNA polymerase or by restriction digestion.
- Confirm and determine concentration of fragments and linearized vector using agarose gel electrophoresis, a NanoDrop™ instrument or other method.
- Add fragments and linearized vector to Gibson Assembly Master Mix and incubate at 50°C for 15 minutes to 1 hour, depending on number of fragments being assembled.
- Transform into NEB 5-alpha Competent E. coli (provided) or use directly in other applications.
| Price | 1.362,00 RON (preturile sunt fara TVA) |
|---|---|
| Description |
Gibson Assembly® allows for successful assembly of multiple DNA fragments, regardless of fragment length or end compatibility.
Specification: 10 μl of 2X Gibson Assembly Master Mix was incubated with 6 fragments (5 fragments of 400 bp and one of 2,780 bp, with 40 bp overlap, 0.05 pmol each) in a final volume of 20 μl at 50°C for 60 minutes. NEB 5-alpha Competent E. coli (NEB #C2987) were transformed with 2 μl of the master mix/fragment mixture using the transformation protocol on page 12. Greater than 100 white colonies were observed when 1/10 of the outgrowth was spread on an ampicillin plate with IPTG/Xgal and incubated overnight.
|

 English
English